Program Director:
Co-Program Directors:
Program Administrator:
Peter Davies, MD, PhD
Professor & Director, Center for Translational Cancer Research
Institute of Biosciences & Technology, Texas A&M University
Suzanne Tomlinson, PhD
Director, Research Programs and Strategic Initiatives,
Gulf Coast Consortia
Zhiqiang An, PhD
Professor and Robert A. Welch Distinguished University Chair in Chemistry
Brown Foundation Institute of Molecular Medicine
Director, Texas Therapeutics Institute
University of Texas Health Science Center at Houston
Wenshe Ray Liu, PhD
Professor and Gradipore Chair in Chemistry
Director of the Texas A&M Drug Discovery Laboratory
Texas A&M University
Elizabeth Lawrence
Program Administrator, Gulf Coast Consortia
Karen Ethun
Executive Director, Gulf Coast Consortia/Keck Center
CTTP Information Session – June 8, 2021
(click to download slides)

Or click HERE to watch the recorded presentation
The Cancer Therapeutics Training Program (CTTP) is a multi-institutional post-doctoral training program designed to prepare post-doctoral trainees for future careers in academic and/or commercial cancer therapeutics research and development (R&D). The goal of the program is to recruit and train scientists equipped with the essential skills and knowledge necessary to translate basic cancer research discoveries into commercially viable cancer therapeutics.
The CTTP is a partnership between the Gulf Coast Consortia (GCC) and four partner institutions:
- Institute of Biosciences and Technology (IBT) / Texas A&M University (TAMU)
- University of Texas Health Science Center – Houston (UTH)
- Texas Southern University (TSU)
- Baylor College of Medicine (BCM)
The CTTP will leverage the outstanding resources, the large pool of talented trainees and the large number of faculty engaged in cancer therapeutics research in the Gulf Coast Consortia to create an innovative new postdoctoral training program. This training program is designed to leverage CPRIT’s investment in cancer research and cancer research infrastructure and serve as the foundation for a training program that will equip and prepare the next generation of scientists to translate scientific breakthroughs into new cancer therapeutics.
The goal of the CTTP is to build upon a core of mentored training focused on the practical aspects of cancer therapeutics research, coupled with a broad-based instructional program on the foundational principles involved in the discovery, development and commercialization of therapeutics. The Training Plan includes a robust professional development program that will prepare trainees for productive careers in cancer therapeutics research in academia, government or the private sector.
The Cancer Therapeutics Training Program (CTTP) addresses the critical need in Texas for a cadre of trained scientists equipped with the essential skills and knowledge necessary to translate basic cancer research discoveries into commercially viable cancer therapeutics. The CTTP will expand the current GCC Innovative Drug Discovery and Development (IDDD) educational program and will provide postdoctoral fellows with training opportunities that include: 1) training in the fundamentals of drug discovery research; 2) hands-on training in experimental technologies; 3) opportunities for networking and collaboration and 4) career development opportunities.
Trainee Eligibility and Level
- Must be a postdoc in a GCC member institution or one of the 4 CTTP partner institutions and live in and conduct their research in Texas during the training.
- Must have completed their doctoral degree and be engaged in postdoctoral training (ideally 1st, 2nd or 3rd year of training) by the application due date.
- May be US Citizen, Permanent Resident, or Foreign National with a current work visa. (Work visa must be valid for entire appointment period)
- Must agree to participate in the CTTP curriculum, including the 1 week Foundations of Cancer Therapeutics (FCT) “Crash Course.”
- Trainees will be expected to identify both a primary mentor and at least one co-mentor:
-
- If a postdoc is applying for a CTTP CPRIT Fellowship, the mentor must be from a CTTP partner institution; the co-mentor, from the CTTP faculty (email) list.
- If a postdoc is applying for a CTTP Traineeship, both mentor and co-mentor must be from a GCC and/or CTTP partner institution.
Justified exceptions are allowed for both cases.
-
- In addition, trainees from the CTTP Partner Institutions (IBT/TAMU, UTH, TSU, or BCM) will be eligible to compete for CPRIT-funded CTTP fellowship awards at the NRSA-recommended stipend level.
Cancer Therapeutics Training Program
Includes three major components:
- Research Training Program (RTP) will provide trainees with supervised training in the practical aspects of cancer drug discovery research and development. The mainstay of the RTP will be the conduct of one or more mentored research projects in cancer therapeutics research. The RTP will also include one or more rotations in the partnering CPRIT-funded Core Labs to provide trainees with hands-on training in the technologies and conceptual issues involved in drug discovery and development research.
- The Instructional Curriculum (IC) will provide trainees with foundational knowledge on cancer therapeutics, drug discovery and development, and the therapeutics commercialization. All trainees will be expected to participate in the mandatory components of the CTTP curriculum and will have the opportunity to participate in voluntary CTTP educational programs.
-
- Mandatory Components include:
- Intensive 1-week Foundations of Cancer Therapeutics (FCT) Crash Course (TBD – August 2025) that surveys key elements in the process of discovering, developing and commercializing cancer therapeutics. NOTE: Those accepted into CTTP MUST attend this course. More information about FCT is in the Curriculum section below.
- Participate in at least one 4-8 week “rotation,” (15-20 hrs per week) in core laboratories or “internship” in collaborating Tech Transfer Offices and Companies engaged in commercial aspects of therapeutics R&D.
- CTTP trainees will complete three mandatory training modules over the course of a 2-year period: 1) Responsible Conduct of Research (RCR), 2) Rigor and Reproducibility and 3) Diversity, Disparities and Community Engagement that address key areas of ethical and social responsibility for scientists engaged in biomedical research. Trainees who have completed prior training in one or more of these or comparable training modules may be exempted from this requirement.
- Join monthly Gulf Coast Consortia (GCC) Innovative Drug Discovery and Development (IDDD) Roundtable workshops on the 2nd Thursday of each month.
- Participate in CTTP Careers Roundtables for at least one annual cycle of these monthly presentations.
- Attend a minimum of 2 career/skill building workshops each year.
- Trainee/Mentor teams will develop and submit IDPs.
- In addition to participation in the CTTP Research Mini-Symposia and the Keck Annual Research Conference, all CTTP trainees will be encouraged to present their research at the annual IDDD conference.
- Mandatory Components include:
-
- Optional Components include:
- Trainees will have the option of selecting advanced elective graduate courses available through the graduate programs of the participating CTTP institutions.
- Optional Components include:
-
- The Career Development Program (CDP) will provide trainees with information, experience and professional skills training that will contribute to their future success as leaders in either academic or private-sector cancer therapeutics R&D. The CDP will include a series of roundtables, workshops and networking opportunities that will familiarize trainees with the full range of career opportunities available within the field of cancer therapeutics research.
The Gulf Coast Consortia is committed to providing equal opportunity in training for individuals with disabilities and individuals from racial and ethnic groups who are currently under-represented in STEM fields. We welcome applications from all qualified trainees, regardless of ethnicity, race, or disability status. All GCC member institutions are ADAAA compliant and have offices of disability support services that provide accommodations and support services to trainees, faculty, staff, and visitors.
Click here to download the CTTP trainee application form
Components of the CTTP trainee application
All application material, including recommendation letters, must be submitted to Elizabeth Lawrence, CTTP Administrator (el53@rice.edu) by October 28, 2024. The application is a 2-part process. All trainees applying for acceptance into the CTTP will be required to provide the following:
- Fellowship Application form (Click Here). Components besides educational background and published papers include:
- Project description
- Mentoring and Training Plan
- Career Goals
- CV or resume, including list of Publications and Presentations. Email to Elizabeth Lawrence el53@rice.edu a current resume or CV outlining your professional work experience and academic history, including the dates you received your degree(s).
- Primary Mentor Recommendation Letters. (Mentor and co-mentors must follow the below format when preparing their respective support letters. Mentors are to email their letters directly to Elizabeth Lawrence, CTTP Administrator (el53@rice.edu).
-
- Describe your role in the applicant‘s proposed research project. Evaluate the applicant’s overall ability and likelihood of becoming a productive researcher in the field of cancer therapeutics research.
- The mentor’s current NIH Biosketch and Other Support document.
- Co-Mentor(s) Support Letter
-
- Support letter should include a description of the co-mentor’s involvement with this project.
- The Co-mentor’s current NIH Biosketch and Other Support document.
Additional instructions for those applying for CPRIT CTTP Fellowships
Applications will also include the following
- Two (2) additional Letters of Recommendation from people other than your mentors. These letters may be from former professors, employers, etc. The recommenders should email them directly to Elizabeth Lawrence el53@rice.edu.
- For Non-US Citizens, documentation of work visa status is required. Please note that the work visa must be valid for the entire appointment period.
Interview:
Applications will be reviewed, and selected applicants for CPRIT CTTP fellowships may be invited to interview with the Program Directors and the CTTP Steering Committee November 14, 2024.
All applicants will be notified via email whether or not they have been selected for an interview. Interviews are a total of 15 minutes (8-10-minute presentation + Q&A) and are held within one month of the application deadline. Interviews include a brief presentation of the research project, planned coursework, and mentoring plan. Mentors (primary and co-mentors) are expected to attend the interview in support of their trainee. (See the section Instructions for Interviews for details about limits on time and number of slides).
Please also note that there is a quick turn around for interviews after applicants have been selected. Applicants are encouraged to start working on their slides and interviews as soon as they turn in their applications.
Selected applicants and their mentors will be invited for an interview before the CTTP Program Directors and Steering Committee. All interviews will take place on one day November 14, 2024. Mentors are expected to attend. All of the below should be in your presentation. Please also note that there is a quick turn around for interviews after applicants have been selected. Applicants are encouraged to start working on their slides and interviews as soon as they turn in their applications.
Prepare a brief oral presentation:
- 10 minutes maximum, with a maximum of 6 PPT slides + title slide + acknowledgment slide.
- Do not embed movies in your slides.
- The interview will last a total of 15 minutes, including your 8-10 minute oral presentation, then questions from the reviewers.
- Please practice so that you do not exceed this time limit (hint: do not get bogged down in the details of the experiments).
- Aim to keep your presentation simple; you will be assessed on how well you can explain things concisely to a broad audience, as the CTTP Steering Committee includes faculty from various disciplines related to Cancer Therapeutics.
Your presentation should provide an overview of your proposed research project and should address the following:
- the relevance of your project to the overall process of cancer therapeutics R&D. This does not need to be lengthy or highly detailed, but it should be explicit. Oftentimes this may be obvious to you and others in your specific area, but it may not be clear to a diverse review group.
- the roles that both your primary mentor and co-mentors (e.g. core directors of labs through which candidate will rotate) will play in the proposed research / mentoring plan, especially how the mentors together will enable you to conduct research that could not be accomplished otherwise.
- brief statement about how being a CTTP trainee aligns with your career interests and aspirations, i.e. how will completing the CTTP fellowship and programs requirements lead you to a fulfilling career in cancer therapeutics
Foundations of Cancer Therapeutics (FCT) Crash Course. The goal of the FCT Crash Course is to serve as an efficient and effective way of providing all CTTP trainees with a survey of the foundational concepts necessary for successful careers in cancer therapeutics R&D. The FCT will be structured as an intensive blend of didactic learning modules and facilitated discussions focused on three major topics: 1) Fundamentals of Cancer Therapeutics, 2) Fundamentals of Drug Discovery and Development, 3) Fundamentals of Biotechnology and Business Development (see Table III). The FCT course will be delivered over 1 week in Summer 2025 (TBD August 2025) prior to the start of the academic year. The FCT will be capped with a CTTP Research Symposium featuring presentations by CTTP trainees and their mentors.
Those accepted into CTTP MUST attend this course August 2025.
CTTP Electives Program. The goal of the CTTP Electives Program is to provide trainees with access to specialized graduate courses that may be relevant to their research programs and aligned with their professional interests. CTTP trainees will come from diverse academic backgrounds and will be pursuing diverse aspects of cancer therapeutics R&D so the CTTP will provide trainees with the maximum flexibility to develop their own instructional curriculum based on their individual interests and requirements (to be included in the Independent Development Plan submitted during the FCT course). The CTTP offers a rich “menu” of courses in cancer biology and pharmacology / drug discovery that will allow trainees to construct a personalized curriculum of specialized training. CTTP postdoctoral fellows will participate as non-degree registrants (with permission of the instructor).
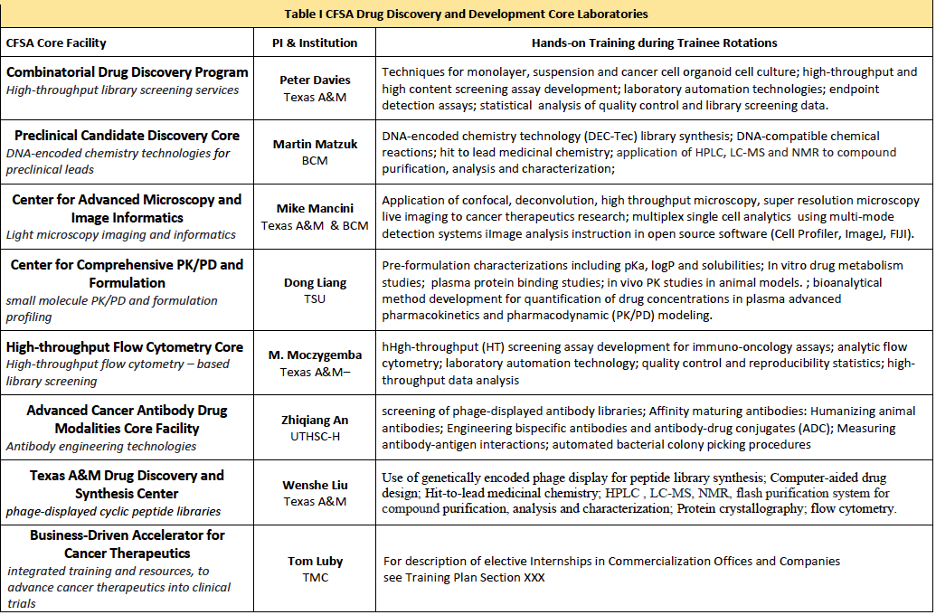
Core Facility Rotations and Internships Program. The goal of the Rotations and Internships Program is to provide CTTP trainees with hands-on training on the research technologies and research procedures involved in drug discovery and development research. The GCC IDDD is home to a network of CPRIT-funded CFSA Core Laboratories that are superbly equipped to provide trainees with access to the full range of experimental technologies involved in drug discovery and development research. Trainees will participate in at least one 4-8 week (15 – 20) hrs per week, “rotation” in core laboratories or “internship” in collaborating Tech Transfer Offices and Companies engaged in commercial aspects of therapeutics R&D. Rotations and internships will last for 4 – 8 weeks and will be structured to provide trainees the opportunity to be fully immersed in the practical aspects of the core facilities / companies operations and services. The table below provides a summary of the “hands-on” learning activities that will be gained from participation in core lab rotations in each of the participating programs.
The steering committee review and select candidates for appointment to CTTP and: 1) provide input into the direction, philosophy and implementation of the training activities, 2) to monitor the educational programs and progress of selected trainees, 3) to interact with program faculty to improve and optimize the curriculum as well as suggest implementation of new courses or educational activities, 4) to review and select candidates and 5) to aid in any conflict resolution. The committee will also meet with each trainee on a yearly basis at their annual progress review.
- Peter Davies, IBT
- Zhiqiang An, UTH
- Scott Gilbertson, UH
- Dong Liang, TSU
- Wenshe Liu, TAMU
- Cliff Stephan, IBT
- Suzanne Tomlinson, GCC
- Stan Watowich, UTMB
- Damian Young, BCM
- TBA, MDA